
Why Choose waveguard touch

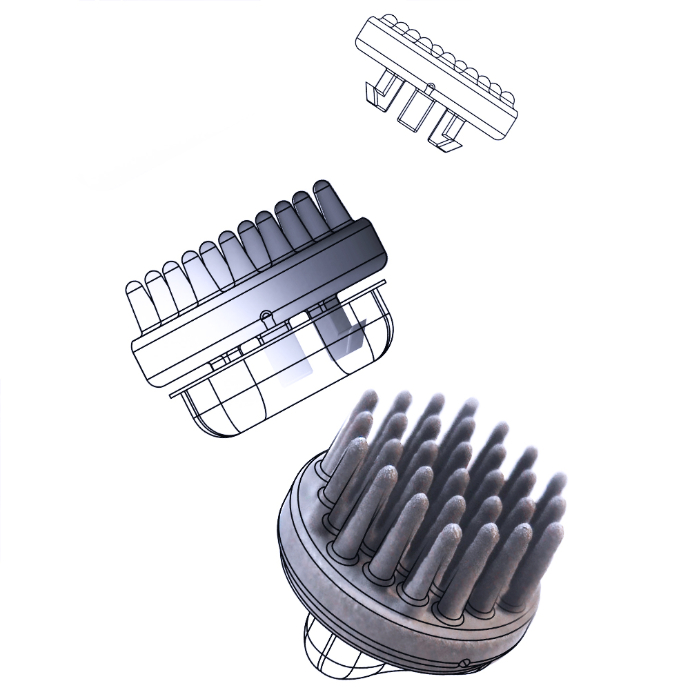
Dry EEG Recording
Stable, research-grade dry EEG recording with coated Ag/AgCl EEG electrodes
Time-Saving Application
Save valuable application time without the need to gel the electrodes

Clean Signal Quality
Clean EEG signals with active shielding technology


Sizes to Fit Everyone
Available in various sizes for different head circumferences and shapes – from neonatal to infants and adults
Unmatched Comfort Design
Unmatched comfort with soft, multi-pin electrode design to consistent contact
Showcases and Publications


Noise characteristics in spaceflight multichannel EEG
By Patrique Fiedler, Jens Haueisen, Ana M. Cebolla Alvarez, Guy Cheron, Pablo Cuesta, Fernando Maestú, Michael Funke.
Read MoreMore
Enhanced Features
Do you have questions or need an offer?
DISCLAIMER: All waveguard™ EEG caps and accessories are CE marked medical devices, according to MDR (EU) 2017/745, CE class I and are registered in the ARTG in compliance with the Australian TG(MD)R. waveguard family of products consist of waveguard original, waveguard connect, waveguard touch, waveguard net caps and waveguard accessories.
Information intended for users in USA/Canada: waveguard original and waveguard connect caps have FDA clearance under 510(k) in the USA and Medical Device License (MDL) issued by Health Canada. waveguard net caps have FDA clearance under 510(k) in the USA. waveguard touch caps are currently for research only in the USA / Canada. IRB approval required for clinical use.
Manufactured by eemagine Medical Imaging Solutions GmbH, Berlin, Germany, ISO 13485 certified. ANT Neuro and eemagine are part of the neuromotion group.
For more information about waveguard EEG caps and accessories and the regulatory status in your country, contact us.



