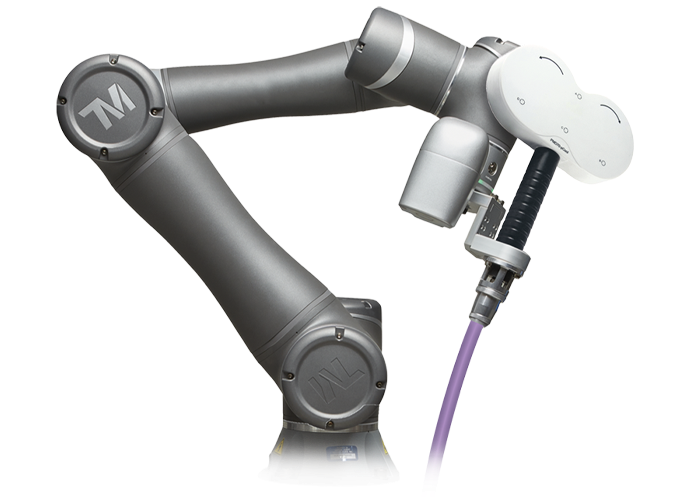
Why Choose visor2
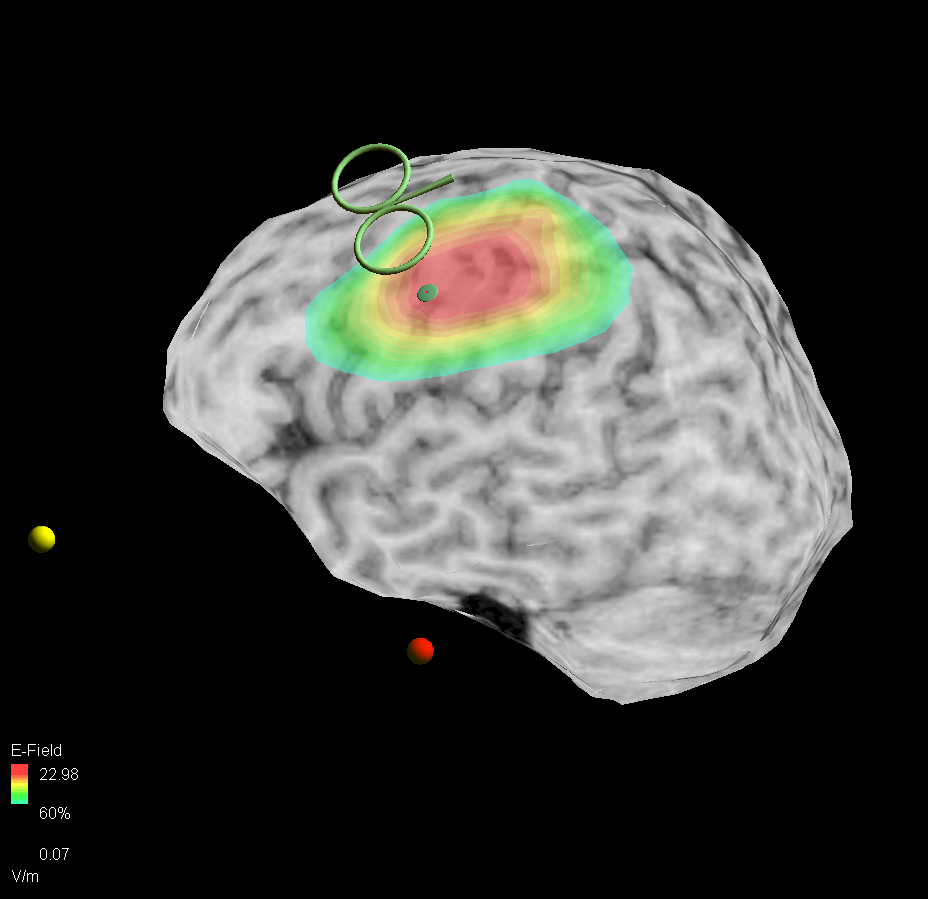
Precise and Consistent Targeting
Precise and reproducible delivery of TMS pulses with state-of-the-art optical tracking technology and individual MRI data
Configurable Neuronavigation
Configurable neuronavigation solution designed for MRI-less or (f)MRI-guided targeting, tailored to meet the specific requirements and needs of the user
User-Friendly Workflow
User-friendly, step-by-step software workflow for ease of use and reduced training effort
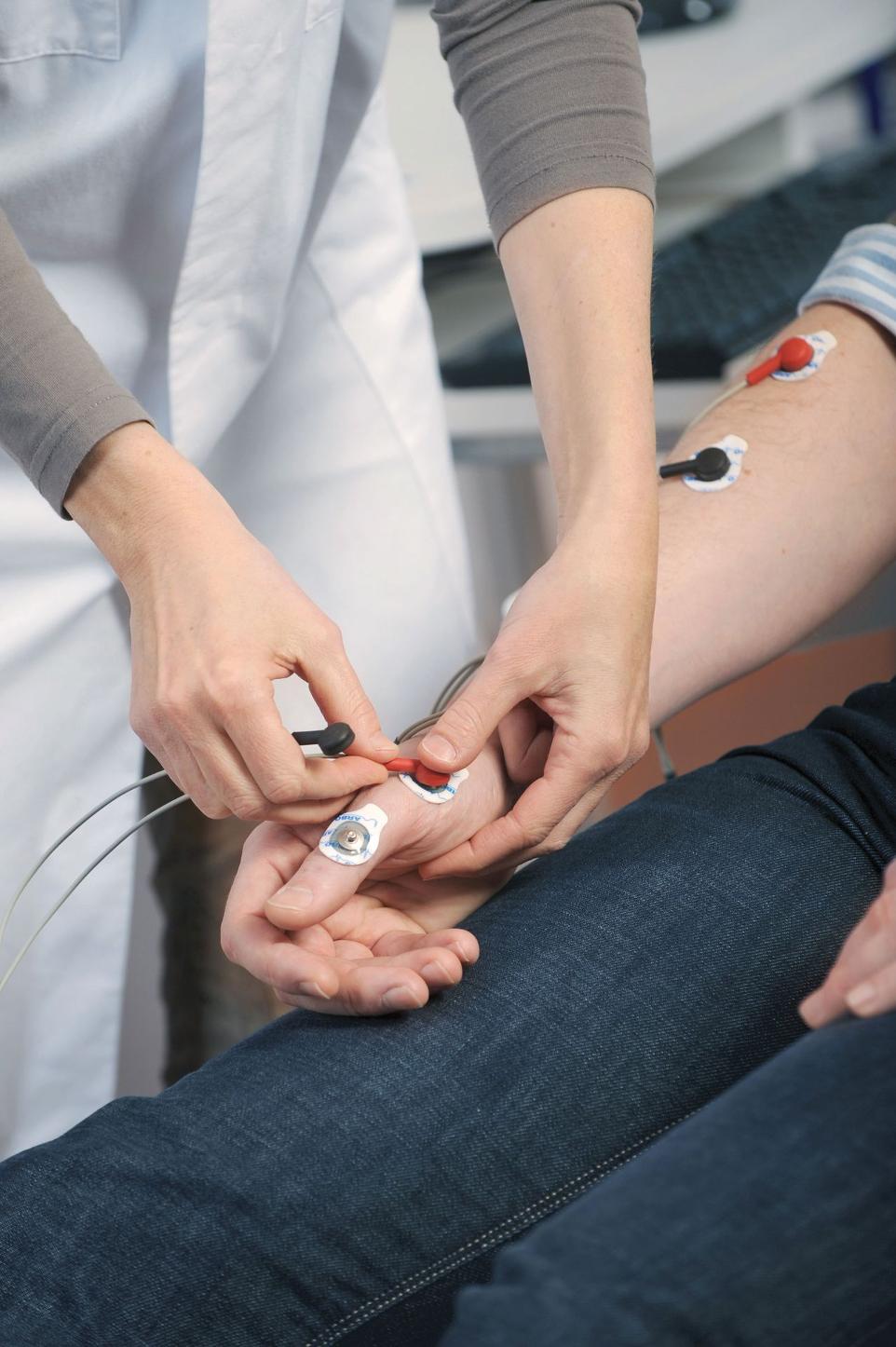
Individual Motor Threshold
Facilitates motor threshold determination with enhanced motor mapping functions and flexible number of EMG channels
Broad TMS Stimulator Compatibility
Compatible with the majority of FDA-cleared TMS stimulators from Mag&More, Magstim, Magventure and Neurostar
Cost-Effective Solution
Provides a cost-effective solution featuring reusable accessories and no recurring costs per session
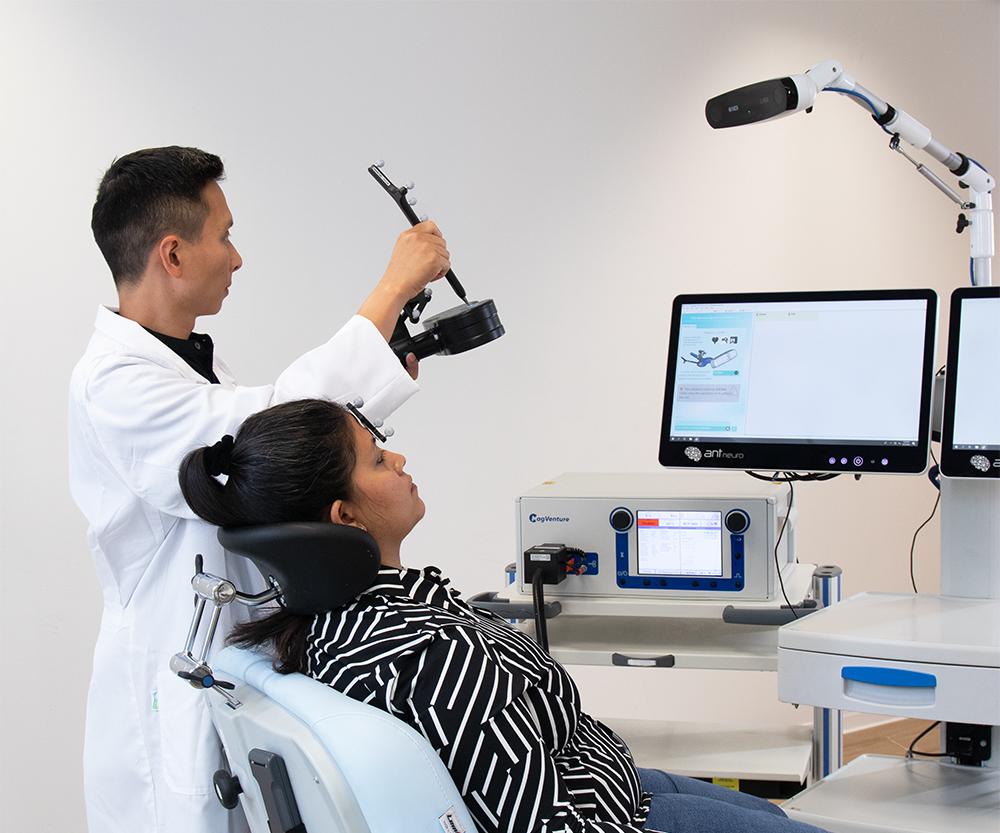
Showcases

Clinical Application of visor2 for Brain Stimulation
Explore the clinical advantages of visor2 with expert insights from Dr. Rebecca Cohen and Dr. Jordana Hollen of Cohen & Associates.
Read MoreEnhanced Features
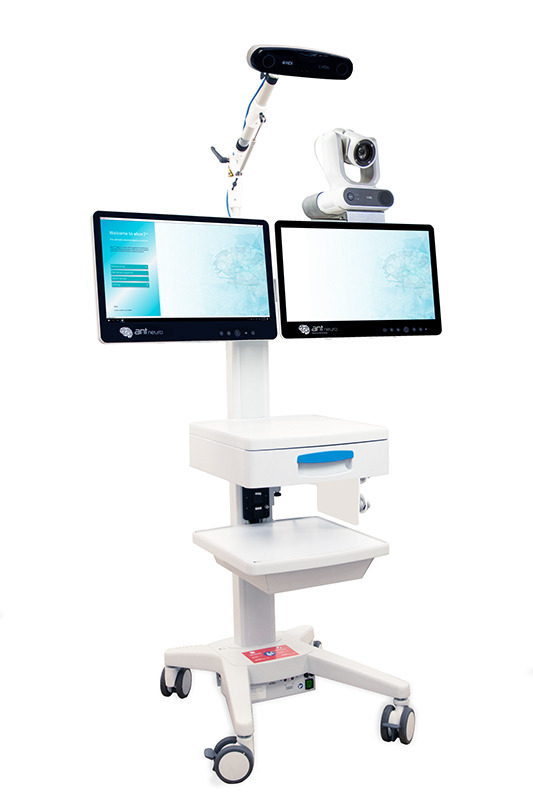
Do you have questions or need an offer?
DISCLAIMER: visor2 system is CE marked as a medical device in the EU, according to MDD 93/42/EEC, class lla and has FDA clearance under 510(k) in the USA. Medical Device License (MDL) issued by Health Canada. Compliant with the Australian TG(MD)R and registered in the ARTG. Manufactured by eemagine GmbH, Berlin, Germany, ISO 13485 certified. ANT Neuro and eemagine are part of the neuromotion group. For the regulatory status of visor2 outside of Australia, Canada, EU and USA, please contact your local distributor or ANT representation. EEG-TMS, language mapping and dual-coil navigation with visor2 is intended for research and educational use only.
Information for United States based clinicians: conditions that are FDA cleared for treatment with TMS are specific to each stimulation device. Please consult your stimulator manufacturer for more information.
*available with the visor2 functional language mapping add-on (investigational use only)
**only available with visor2 professional and premium variants