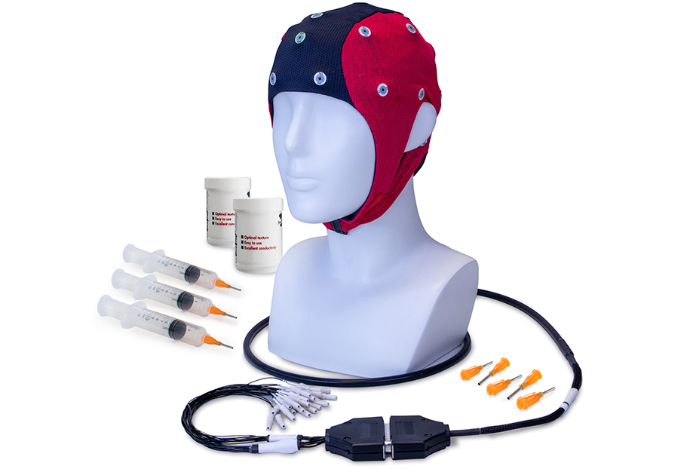
Why Choose waveguard connect
Universal Compatibility
Compatible with all major clinical EEG systems
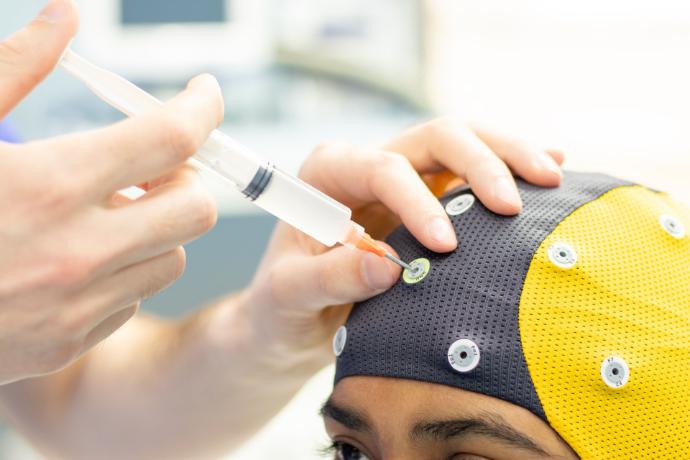
Enhanced Comfort
Patient comfort is ensured during long recordings with soft silicone cups on the electrodes
Optimal Signal Quality
Experience optimal signal quality with reliable tin-based electrodes


User-Friendly Design
User-friendly and low maintenance for effortless operation
Versatile Fit
A few sizes fit all design ensures full coverage for various adult head circumferences
Quick Size Selection
Distinct colored fabrics allow for quick selection of the right cap size (from baby to large)
Publications

Mobile EEG reveals functionally dissociable dynamic processes supporting real-world ambulatory obstacle avoidance: Evidence for early proactive control
Magda Mustile, Dimitrios Kourtis, Simon Ladouce, Gemma Learmonth, Martin G. Edwards, David I. Donaldson, Magdalena Ietswaart.
Read More
High-density EEG in current clinical practice and opportunities for the future
Stoyell, Sally M.; Wilmskoetter, Janina; Dobrota, Mary-Ann; Chinappen, Dhinakaran M.; Bonilha, Leonardo; Mintz, Mark; Brinkmann, Benjamin H.; Herman, Susan T.; Peters, Jurriaan M.; Vulliemoz, Serge; Seeck, Margitta; Hämäläinen, Matti S.; Chu, Catherine J.
Read More
Response inhibition in problematic social network sites use: an ERP study
Tania Moretta, Giulia Buodo
Read MoreEnhanced Features

Do you have questions or need an offer?
DISCLAIMER: All waveguard™ EEG caps and accessories are CE marked medical devices, according to MDR (EU) 2017/745, CE class I and are registered in the ARTG in compliance with the Australian TG(MD)R. waveguard family of products consist of waveguard original, waveguard connect, waveguard touch, waveguard net caps and waveguard accessories.
Information intended for users in USA/Canada: waveguard original and waveguard connect caps have FDA clearance under 510(k) in the USA and Medical Device License (MDL) issued by Health Canada. waveguard net caps have FDA clearance under 510(k) in the USA. waveguard touch caps are currently for research only in the USA / Canada. IRB approval required for clinical use.
Manufactured by eemagine Medical Imaging Solutions GmbH, Berlin, Germany, ISO 13485 certified. ANT Neuro and eemagine are part of the neuromotion group.
For more information about waveguard EEG caps and accessories and the regulatory status in your country, contact us.